Securities code:603520
Good news|Shanghai Starry loversol lnjection approved for marketing
2023-10-31
On 27th October, according to NMPA’s public information, Shanghai Starry declared the Ioversol injection (National Medical Standards H20234353) was approved and obtained the drug registration certificate (Deemed to pass consistency evaluation).
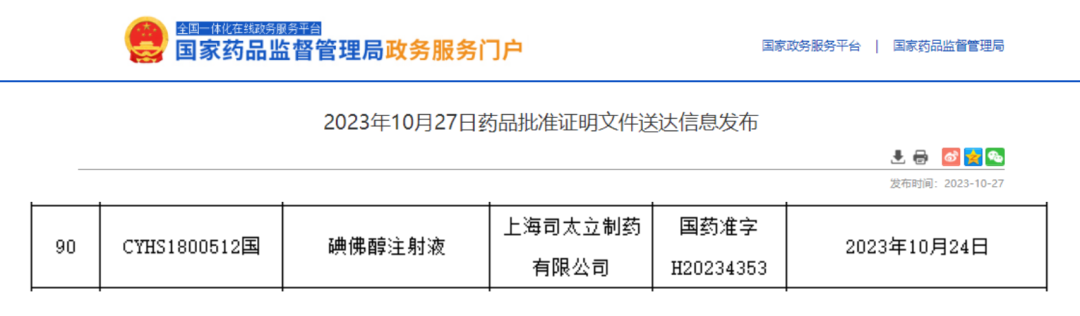
The approval of Ioversol injection is as an important step for Starry to pursue the strategy of whole industry chain for contrast agent. Up to now, Starry has become one of the enterprises with the largest number of approved iodine contrast agents among domestic generic drug enterprises, and the approved products include iohexol injection, iopamidol injection, iodixanol injection, iomeprol injection and ioversol injection. In the future,Starry is firmly committed to this strategy and persisting in buildingup its core competitivenessof API and FDF Integration. Aimed to provide more medicines of high quality to domestic customers, Starry is working hard to further deepening the technological R&D and product innovation in the field of medical imaging.
loversol lnjection
Ioversol injection was firstly developed by Mallinckrodt Company in the United States, which belongs to a kind of non-ionic, low-permeability, water-soluble, monomer X-ray contrast agent. Ioversol injection was approved by the FDA in 1988 and listed on the market in the U.S.A. under the trade name of "Optiray". It entered the Chinese market in 1999, entered the category B of medical insurance in 2000 and category A of medical insurance in 2009.
Related news
Copyright © Zhejiang Starry Pharmaceutical Co., Ltd. All Rights Reserved.
Powered by www.300.cn


Wap
CONTACT US
Tel: 0576-87718628 / 021-37916067
E-mail: sales@starrypharma.com
Zhejiang Starry Pharmaceutical Co., Ltd.
Add: No.l Starry Road of Xianju, Modern IndustriaL, Centralization Zone, Xianju, Zhejiang, China
Shanghai Starry Pharmaceutical Co., Ltd.
Add: No.500 Maoye Road,Jinshan District 201506, Shanghai city, China
Zhejiang Taizhou Hisyn Pharmaceutical Co., Ltd.
Add: Chemical and Medical Raw Materials Base, Linhai Park, Zhejiang, China
Jiangxi Starry Pharmaceutical Co., Ltd.
Add: North of the Wuyl Road,Salt chemical Base, Zhangshu, Jiangxi, 331200, P.R.China
Imax Diagnostic Imaging Limited
Add: Phoenix House, Room 137 Monahan Road, CorkT12H1XY Republic of lreland
E-mail:info@imaxdiagnostic.com
Tel: 00353 21 451 9990
REQUEST A QUOTE






